Our lab’s research focuses on elucidating how memory is encoded, retained, and retrieved within
neural networks and molecules in the brain. We employ and develop cutting-edge tools to record
and manipulate neuronal and molecular activities to unravel neural mechanisms underlying
memory processes. Our integrative approach combines electrophysiology, imaging, behavior,
genetic manipulations, omics, and computational modeling.
The major tools used in the lab include in vivo electrophysiology, two-photon calcium imaging, one-photon endoscopic imaging, fiber photometry, opto-/chemo-genetic manipulations, and ex vivo electrophysiology. We also actively develop our own tools to address specific scientific questions through bioengineering, chemical synthesis, (nano)materials engineering, and electrical engineering.
The major tools used in the lab include in vivo electrophysiology, two-photon calcium imaging, one-photon endoscopic imaging, fiber photometry, opto-/chemo-genetic manipulations, and ex vivo electrophysiology. We also actively develop our own tools to address specific scientific questions through bioengineering, chemical synthesis, (nano)materials engineering, and electrical engineering.
Elucidating neural networks supporting memory encoding and consolidation
See how we identified a hypothalamic novelty hub and how it modulates different types of memories in the hippocampus.
Much of our work focuses on the hippocampus, a brain region giving rise to neural sequences
underlying episodic memory. These sequences, occurring during both behavior (e.g. theta
sequences) and sleep (e.g. replay sequences), lay the foundation for memory encoding and
consolidation. We believe what drives these sequences is a brain-wide oscillation network, and
our lab is elucidating the key nodes in this network and how information is transmitted and
computed among them.
One recent focus of the lab is an understudied subcortical structure in the brain’s oscillation network, the supramammillary nucleus (SuM). This small hypothalamic nucleus is unique in its broad, extensive connections to the hippocampus and cortex. We are investigating how these long-range, bottom-up pathways modulate network dynamics to process memory.
One recent focus of the lab is an understudied subcortical structure in the brain’s oscillation network, the supramammillary nucleus (SuM). This small hypothalamic nucleus is unique in its broad, extensive connections to the hippocampus and cortex. We are investigating how these long-range, bottom-up pathways modulate network dynamics to process memory.
Whole-brain mapping of SuM projections by clearing.
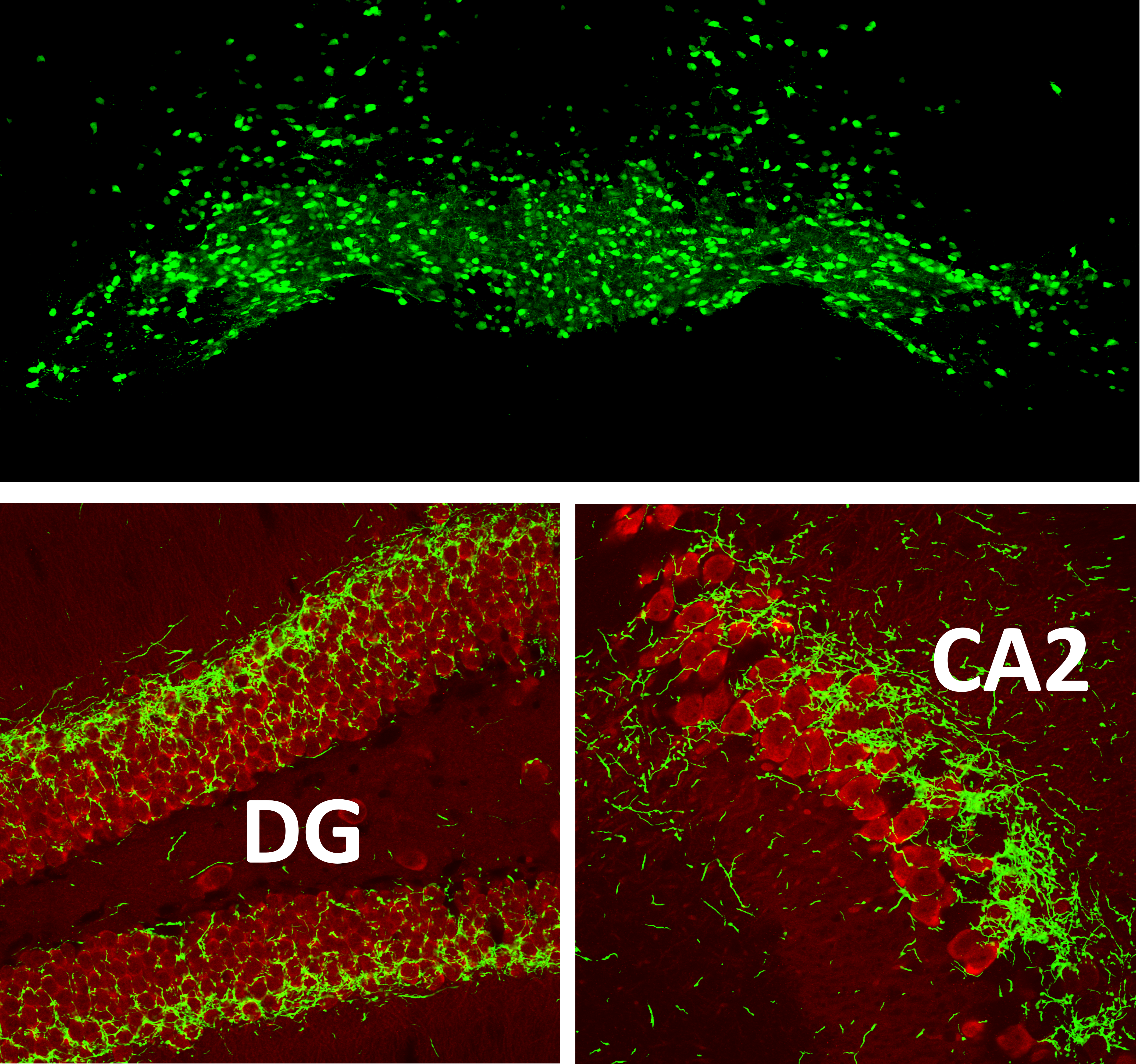
SuM soma (top) and axons in the hippocampus (bottom).

Optogenetic identification and recording of DG- (top) and CA2-(bottom) projecting SuM neurons during behavior.
Deciphering neural computation underlying memory-guided behavior
Complex cognitive activities, such as thinking and planning, rely on established knowledge
about the external world. We believe that through elaborately designed behavioral tasks
combined with large-scale in vivo recordings of neural activity, we may probe the underlying
neural computation mechanisms.
Our lab employs this approach to investigate how the brain synthesizes novel ideas and solutions from stored information–i.e. memory retrieval and utilization. By drawing inspiration from psychology, engineering, and computer sciences, we aim to dissect neural computational mechanisms underlying memory-guided complex behaviors.
Our lab employs this approach to investigate how the brain synthesizes novel ideas and solutions from stored information–i.e. memory retrieval and utilization. By drawing inspiration from psychology, engineering, and computer sciences, we aim to dissect neural computational mechanisms underlying memory-guided complex behaviors.
Goal-directed spatial navigation in T-maze.
Developing next-generation, noninvasive brain machine interfaces for memory and cognitive control
See
how we developed and showcased a minimally invasive technology,
near-infrared upconversion optogenetics, for various neuromodulation applications,
e.g. recalling an episodic memory by transcranial NIR light.
Manipulating memory and cognition requires technologies to spatiotemporally modulate nervous
systems in deep tissue, sometimes in a precision closed-loop manner. For decades, physicians
have utilized implanted electrodes connected through wires to a pacemaker-like device under the
skin of the chest to electrically stimulate neurons deep in the brain. This approach, known as
deep brain stimulation, has proven to be effective, but is costly, highly invasive, and lacks cell
specificity.
Our lab is developing noninvasive, high-precision neuromodulation technologies to manipulate diverse deep structures in the nervous system that are conventionally hard and intrusive to reach. We adopt a cross-disciplinary approach by designing and synthesizing molecularly engineered, noninvasively delivered nano- or micro-interfaces capable of converting tissue-penetrable stimuli into signals recognized/sensible by genetically defined cells. We believe such efforts will contribute to the field’s collective efforts in unlocking diverse routes towards next-generation, noninvasive neuromodulation therapies.
Our lab is developing noninvasive, high-precision neuromodulation technologies to manipulate diverse deep structures in the nervous system that are conventionally hard and intrusive to reach. We adopt a cross-disciplinary approach by designing and synthesizing molecularly engineered, noninvasively delivered nano- or micro-interfaces capable of converting tissue-penetrable stimuli into signals recognized/sensible by genetically defined cells. We believe such efforts will contribute to the field’s collective efforts in unlocking diverse routes towards next-generation, noninvasive neuromodulation therapies.
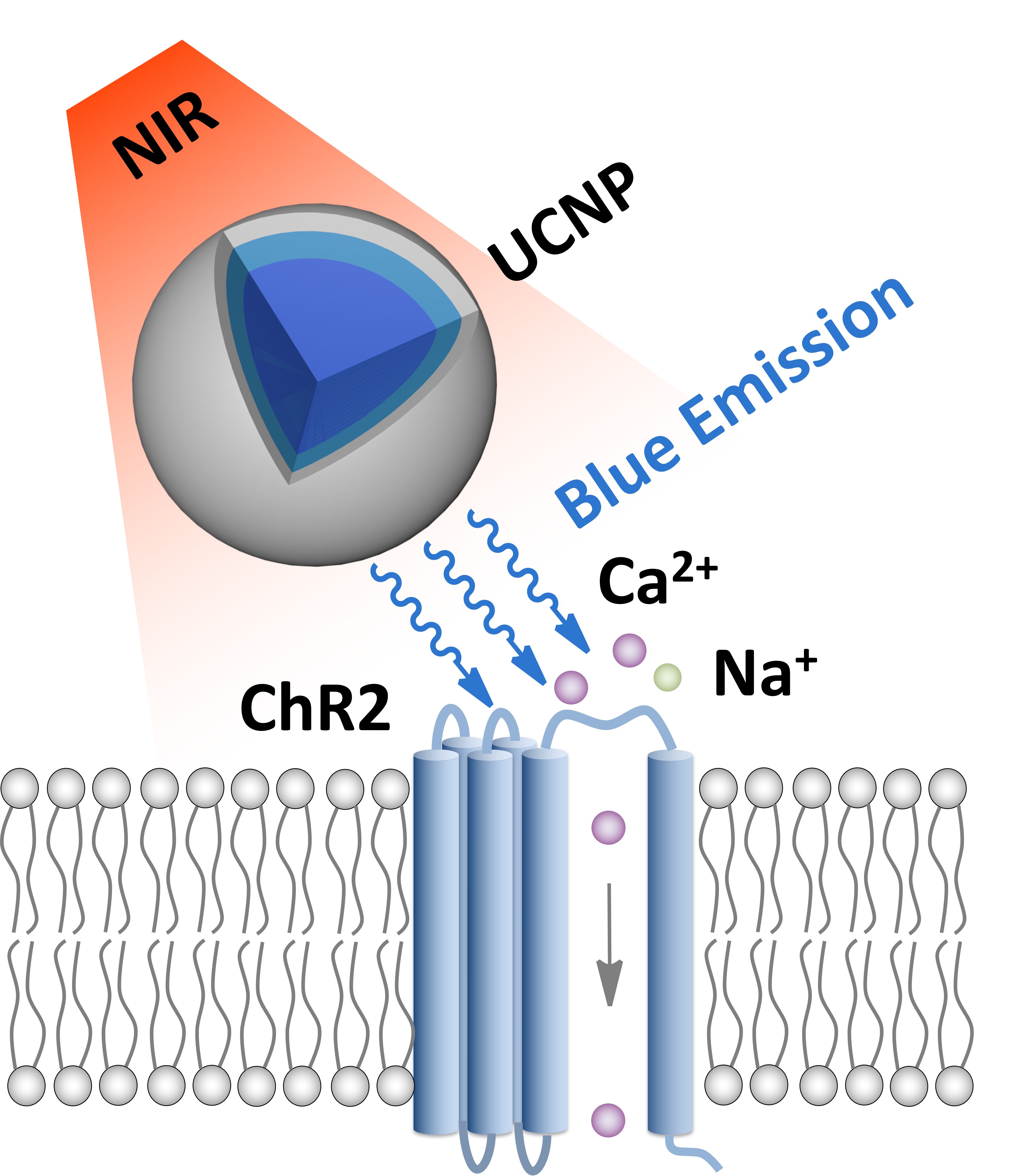
Near-infrared optogenetics mediated by upconversion nanoparticles (UCNPs).
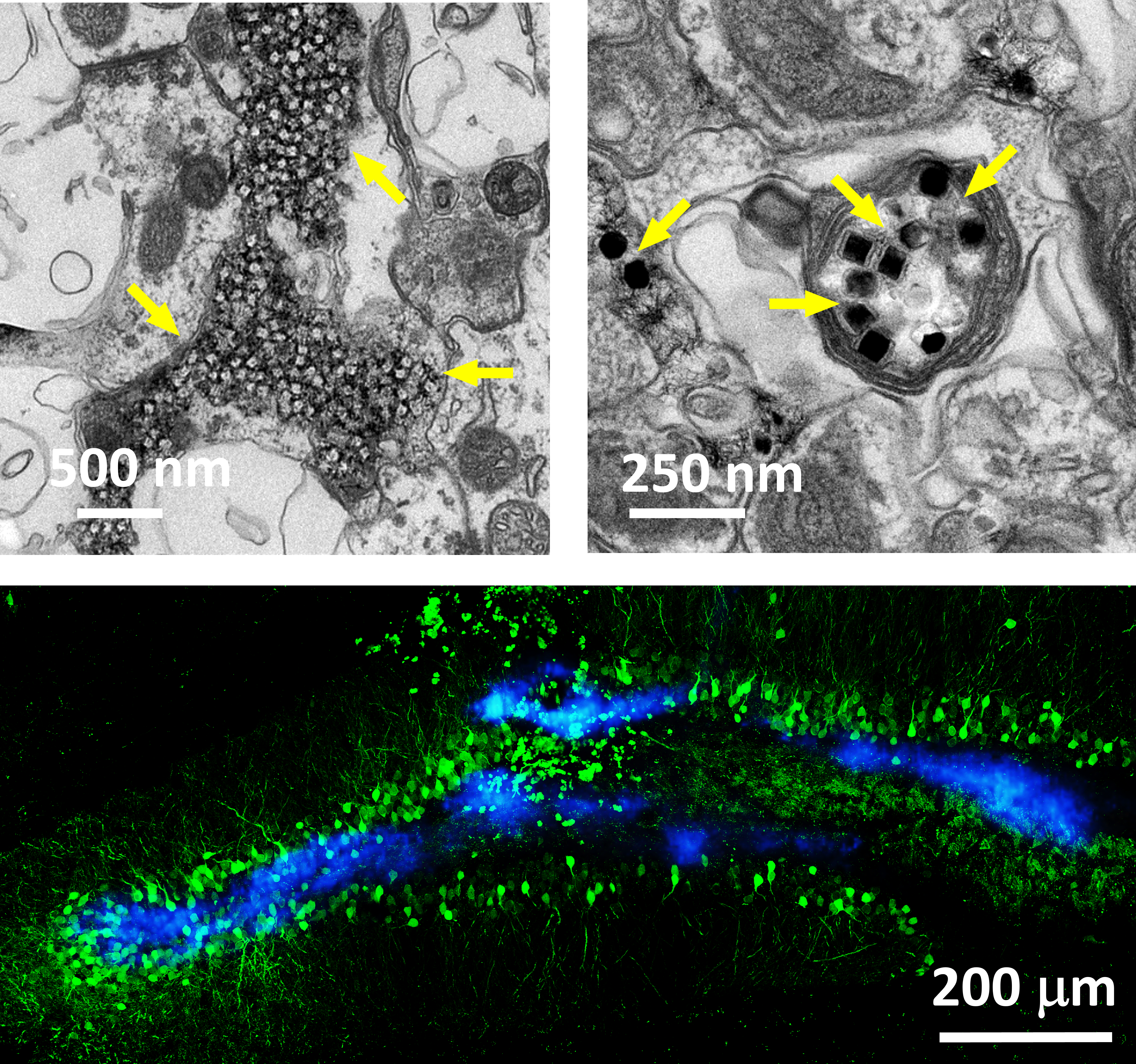
Electron micrographs of UCNPs distributed in tissue (top). Reactivation of hippocampal engram (green) by UCNPs (blue).
Real-time imaging of retrograde axonal transport of UCNPs under a total internal reflection fluorescence (TIRF) microscope.
Linking molecular and network pathologies of Alzheimer’s disease
See how we demonstrated the efficacy of a peptide drug that rescues synaptic
function and cognitive function in a mouse model of advanced-stage AD.
Alzheimer's disease (AD) is the most prevalent cause of dementia in the elderly. There is
currently no cure for AD, and clinical trials only provide modest benefits.
While characterized by pathological molecules like amyloid-β, tau and APOE4, AD is a mental and cognitive disorder. How the molecular pathologies of AD lead to cognitive decline remains elusive. Bridging this gap requires understanding neural circuit mechanisms. By leveraging our expertise in systems neuroscience, our lab is identifying circuit pathophysiology of AD by using animal models of different pathologies.
Once elucidated, circuit mechanisms of AD could be employed to develop next-generation, circuit-targeted therapies to restore memory and cognition. Individualized, circuit-oriented diagnosis and treatment may become possible with time.
While characterized by pathological molecules like amyloid-β, tau and APOE4, AD is a mental and cognitive disorder. How the molecular pathologies of AD lead to cognitive decline remains elusive. Bridging this gap requires understanding neural circuit mechanisms. By leveraging our expertise in systems neuroscience, our lab is identifying circuit pathophysiology of AD by using animal models of different pathologies.
Once elucidated, circuit mechanisms of AD could be employed to develop next-generation, circuit-targeted therapies to restore memory and cognition. Individualized, circuit-oriented diagnosis and treatment may become possible with time.
In vivo calcium imaging using a head-mounted endoscope to probe hippocampal neural dynamics in the AD brain.
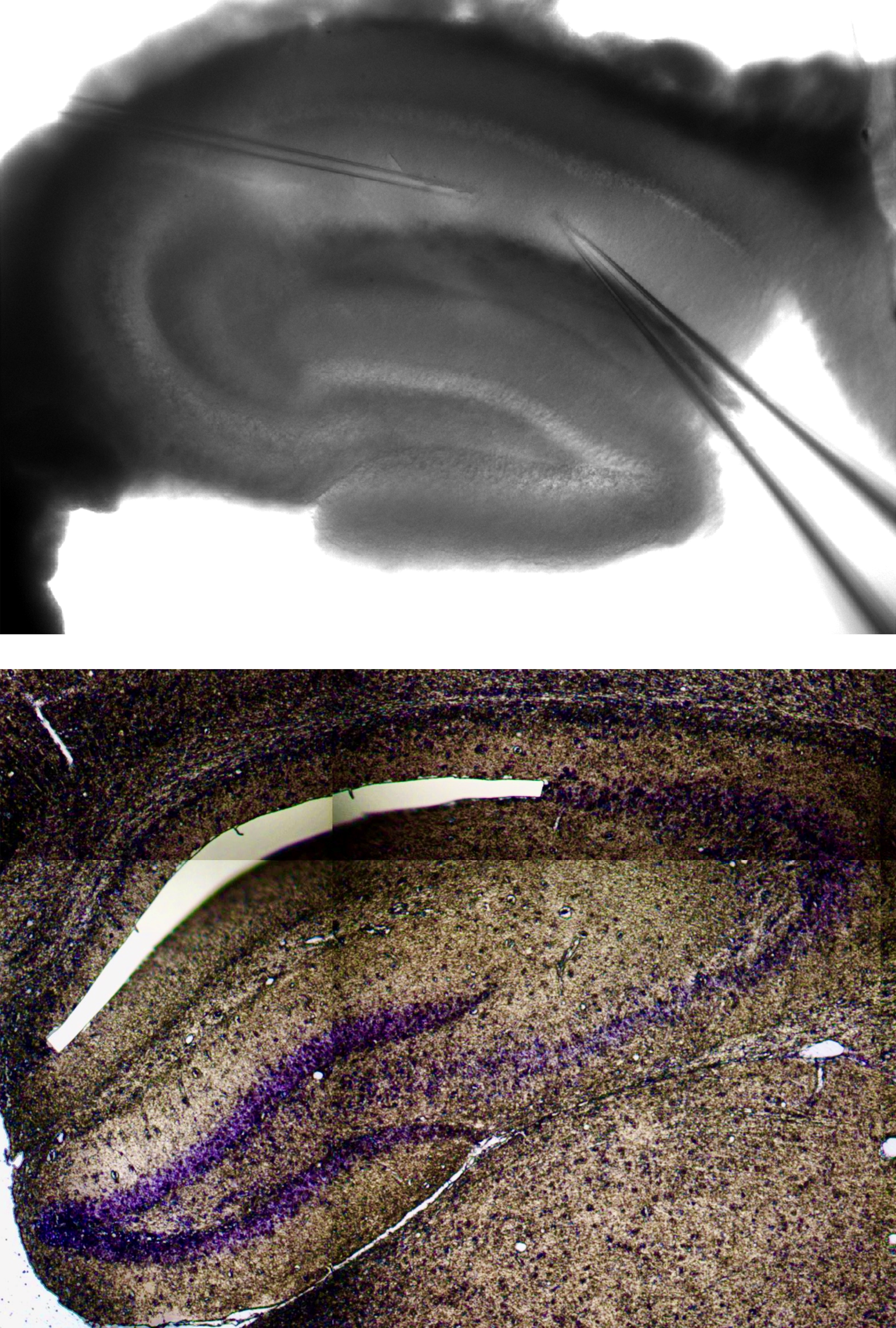
In vitro electrophysiology to measure drug-modified synaptic plasticity.
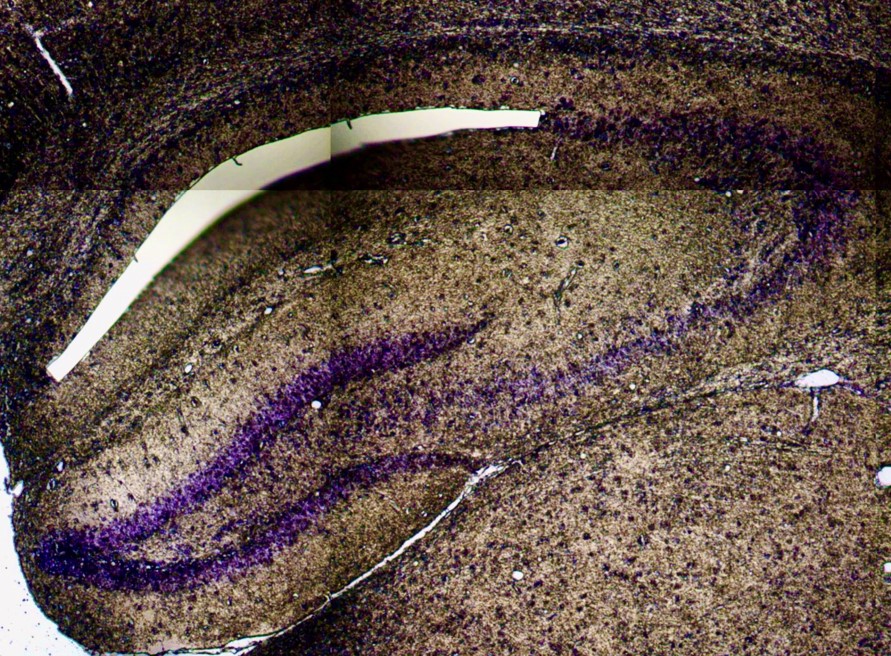
Laser capture microdissection of CA1 cell bodies for localized proteomics.